Characterization of Natural Compounds Produced by Bacillus subtilis Using GC-MS and Investigation of Its antimicrobial activity
Keywords:
Bacillus subtilis, Secondary metabolites, Antibacterial, GC/MSAbstract
Aims and Objectives: Bacterial metabolites have shown promise in the
treatment of a wide range of disorders, and they play an essential role in maintaining
human health. The purpose of this research was to find out how effective Bacillus
subtilis bioactive chemical compounds are against bacteria and other microbes.
Methods: The bioactive chemical components, also called secondary metabolites,
were analysed using gas chromatography-mass spectrometry (GC-MS) methods.
Then, in vitro evaluation of the antibacterial activity of the Bacillus subtilis
methanolic extract was carried out.
Results: The GC-MS analysis of Bacillus subtilis detected the presence of the
following: The compounds listed include tert-Butyl 12-aminododecanoate, 1,12-
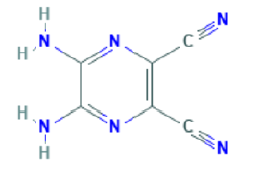
Diaminododecane, Ethylidenehydroxylamine, 5,6-Diamino-2,3-dicyanopyrazine, 1-
methylpiperidine-3-carboxylic Acid, 3,3-Dimethyl-2-acetyloxirane, 8-Hexadecanol,
4-methyl-hexadecane, 5,7-Octadecadiynoic acid, 4,6-dimethylpyridin-2-amine. The
current study examined the bioactivity of the ethanolic extract of Bacillus subtilis
and the standard antibiotics AP-Ampicillin and KN-Kanamycin against five tested
pathogens: Staphylococcus saprophyticus (11.67±0.06, 21.07±0.09, and 12.31
±0.06), Streptococcus pneumonia (12.09±0.07, 22.00±0.08, and 15.86±0.05),
Streptococcus pyogenes (14.51±0.64, 23.05±0.73, and 17.74±0.07). Streptococcus
agalactiae (10.92±0.11, 18.96±0.11 and 18.01±0.09) and Klebsiella pneumoniae
(10.70±0.05, 22.12±0.08, and 19.13±0.09). The metabolites of Bacillus subtilis
exhibited significant activity against Streptococcus pyogenes (14.51±0.64).
Downloads
References
Mora, I., Cabrefiga, J., and Montesinos, E. (2015). Cyclic lipopeptide biosynthetic genes and products, and inhibitory activity of plant-associated Bacillus against phytopathogenic bacteria. PLoSOne 10, e0127738.
Munjal, V., Nadakkakath, A. V., Sheoran, N., Kundu, A., Venugopal, V., Subaharan, K., et al.(2016). Genotyping and identification of broad spectrum antimicrobial volatiles in black pepper root endophytic biocontrol agent, Bacillus megaterium BP17. Biol. Control 92, 66–76. 2015.
Nas, F., Aissaoui, N., Mahjoubi, M., Mosbah, A., Arab, M., Abdelwahed, S., et al. (2021). A comparative GC–MS analysis of bioactive secondary metabolites produced by halotolerant Bacillusspp. isolated from the Great Sebkha of Oran. Int. Microbiol. 24, 455–470.
Raudvere, U., Kolberg, L., Kuzmin, I., Arak, T., Adler, P., Peterson, H., et al. (2019). g: profiler: aweb server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic acids Res. 47, W191–W198.
Shao, Y., Wang, X.-y., Qiu, X., Niu, L.-l., and Ma, Z.-l. (2021). Isolation and purification of a new Bacillus subtilis Strain from deer dung with anti-microbial and anti-cancer activities. Curr. Med. Sci.41, 832–840.
Valli, S., Suvathi, S. S., Aysha, O., Nirmala, P., Vinoth, K. P., and Reena, A. (2012). Antimicrobial potential of Actinomycetes species isolated from marine environment. Asian Pac. J. Trop. Biomed.2, 469–473.
Zhou, M., Liu, F., Yang, X., Jin, J., Dong, X., Zeng, K.-W., et al. (2018). Bacillibactin and bacillomycin analogues with cytotoxicities against human cancer cell lines from marine Bacillus sp.PKU-MA00093 and PKU-MA00092. Mar. Drugs 16, 22.
BonifaitL, Marquis A, Genovese S, Epifano F, Grenier D. 2012. Synthesisand antimicrobial activity of geranyloxy- and farnesyloxy- acetophenonederivatives against oral pathogens. Fitoterapia 83 (6): 996-999.
Devi S, KiesewalterHT, Kovács R, Frisvad JC, Weber T, Larsen TO, Kovács ÁT,Ding L. 2019. Depiction of secondary metabolites and antifungalactivity of Bacillus velezensis DTU001. Synth Syst Biotechnol 4 (3): 142-149.
KumarS, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33 (7): 1870-1874.
Lucena-AguilarG, Sánchez-López AM, Barberán-Aceituno C, Carrillo- Ávila JA,López-Guerrero JA, Aguilar-Quesada R. 2016. DNA source selection for downstream applications based on DNA quality indicators analysis. Biopreserv Biobank 14 (4): 264-270.
Miethke M, Pieroni M, Weber T, Brönstrup M, Hammann P, Halby L,Arimondo PB, Glaser P, Aigle B, Bode HB, et al. 2021. Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem 5 (10): 726-749.
Nas F,Aissaoui N, Mahjoubi M, Mosbah A, Arab M, Abdelwahed S, KhroufR, Masmoudi AS, Cherif A, Klouche-Khelil N. 2021. A comparative GC-MS analysis of bioactive secondary metabolites produced by halotolerant Bacillus spp. isolated from the Great Sebkha of Oran. Intl Microbiol 24 (3): 455-470.
de Oliveira JA,Williams DE, Andersen RJ, Sarragiotto MH, Baldoqui DC.2020. Pumilacidins A-E from sediment-derived bacterium Bacillussp. 4040 and their antimicrobial activity evaluation. J Braz Chem Soc 31 (2): 357-363.
Ong JFM, Goh HC, Lim SC, Pang LM, Chin JSF, Tan KS, Liang ZX,YangL, Glukhov E, Gerwick WH, Tan LT. 2019. Integrated genomic andmetabolomic approach to the discovery of potential anti-quorum sensingnatural products from microbes associated with marine samples from Singapore. Mar Drugs 17 (72): 1-15.
Pham JV, Yilma MA, Feliz A, Majid MT, Maffetone N, Walker JR, KimE, Cho HJ, Reynolds JM, Song MC, Park SR. 2019. A review of themicrobial production of bioactive natural products and biologics.Front Microbiol 10: 1404.
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Current Clinical and Medical Education













