GC/MS Technique for Bioactive Metabolites Analysis of Capsicum frutescens and Evaluation of Its Antioxidant [Superoxide, Nitric Oxide Radical Scavenging] and Antifungal of Four Different Yeast and Fungi
Keywords:
Capsicum frutescens, GC/MS, Antioxidant, Antifungal, MetabolitesAbstract
Background: Despite the fact most of the medicinal plants can be associated with low
mortality, they can lead to severe morbidity such as stunted physical and mental growth, among children
when used in treatment of gastrointestinal diseases such as diarrhoea. Native Americans used capsicum
fruits for various conditions for centuries and the identification of bioactive compounds in these fruits
vindicates this practice. Finding among Capsicum frutescens, our study sought to establish if the plant had
antifungal or antioxidant properties on the basis of its bioactive metabolites.
Methods: The fruits were first washed and then put in an oven at a temperature of 60◦C for 48 hours until
dried. They were then left to cool and later on a fine powder was made from it. The aqueous extract was
obtained after boiling 100mL distilled water with 10g powder at 15 minutes. The preparation of a sample
in a heated injector block and then ongoing on the head of a chromatographic column packed with a non-
volatile liquid phase is the technique called gas chromatography. The inhibitory zone diameter, in
millimeters (mm), is applied as a factor for the evaluation of the result of antifungal efficiency of the
phenolic extracts.
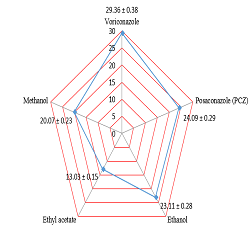
Results: Altogether, GC–MS revealed that Juniperus communis contains more than nineteen wished
natural and helpful secondary metabolites. This paper describes the anti-fungal and anti-yeast actions of
conventional antibiotics and three different ethanolic, methanolic, and ethyle acetate extracts of Juniperus
communis fruits. Phytophthora infestans (16.09 ± 0.19, 13.26 ± 0.15, and 21.19 ± 0.24 respectively), P.
oryzae (20.07 ± 0.23, 13.03 ± 0.15, and 23.11 ± 0.28 respectively), Microsporum canis (19.08 ± 0.22,
15.37 ± 0.18, and 23.94 ± 0.28 respectively), and C. glabrata (13.83 ± 0.15, 21.19 ± 0.24, and 18.01 ±
0.21 respectively). Posaconazole (PCZ) and Voriconazole (VCZ) as standard anti-fungal activity were
(24.09 ± 0.29 and 29.36 ± 0.38) respectively. Capsicum frutescens metabolites was very highly active
against Microsporum canis (23.94 ± 0.28). Antioxidant activity (Superoxide radical scavenging and
Nitric oxide radical scavenging) of (methanol, Ethyl acetate, Ethanol extract and standards) of Capsicum
frutescens. recorded 19.60 ± 1.01, 23.76 ± 1.30, 26.36 ± 1.39 and Quercetin (standard) 39.82 ± 2.07
respectively of Superoxide radical scavenging. 35.00±2.08, 46.51±2.93, 30.28±2.67 and Curcumin
(standard) 83.12±4.07 respectively of Nitric oxide radical scavenging
Conclusion: Bell pepper extracts, like Capsicum frutescens, have antifungal properties against a few
fungal infections that cause food poisoning. There were determined to be biologically active substances,
including alkaloids, flavonoids, polyphenols, and sterols. As a result, Capsicum fruits could be used in
food and medicine as a natural antibiotic. This article provides insight into pepper's use and supports the
use of Capsicum fruit extracts for antifungal activity. The scientific basis for pepper's usage in cooking
and traditional medicine should be strengthened by these findings.
Downloads
References
Dorantes, L.; Colmenero, R.; Hermandez, H.; Mota, L.; Jaramillo, M.E.; Fernandez, E.; Solanco, C. Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annuum extracts. International Journal of Food Microbiology 2000, 57, 125–128.
CCME 2 (7), 107-115 (2024) VISION PUBLISHER|115
Tchiegang, C.; Maoundipa, F.P.; Kapchie, N.V. Etude comparée de quelques constituants chimiques de deux types de piment (Capsicum annuum L.) pendant la conservation dans une saumure acide. Journal of Food Engineering 1999, 42, 117–123.
Boxman, I.; Tilburg, J.; Loeke, N.; Vennema, H.; De Boer, E.; Koopmans, M. An efficient and rapid method for recovery of norovirus from food associated with outbreaks of gastroenteritis. Journal of Food Protection 2007, 70 (2), 504–508.
Nobori, T.; Miurak, K.; Wu, D.J.; Takabayashik, L.A.; Carson, D.A. Detection of the cyclindependent kinase-4 inhibitor gene in multiple human cancers. Nature 1994, 368 (6473), 753–756.
Gopalakrishnan, C.; Shankaranarayan, D.; Kameswaran, L.; Natarajan, S. Pharmacological investigations of tylophorine, the major alkaloid of Tylophora Indica. Indian Journal of Medical Research 1979, 69, 513–520.
Staerk, D.; Lykkeberg, A.K.; Christensen, J.; Budnik, B.A.; Abe, F.; Jaroszewki, J.W. In vitro cytotoxic activity of phenanthroindolizidine alkaloids from Cynanchum vincetoxicum and Tylophora tanake against drug-sensitive and multidrug-resistant cancer cells. Journal of Natural Product 2002, 65 (9), 1299–1302.
Blanco-Ríos A. K., Medina-Juarez L. A., González Aguilar G. A., GamezMeza N. (2013). Antioxidant Activity of the Phenolic and Oily Fractions of Different Sweet Bell Peppers. J Mex Chem Soc 57: 137– 143
Chamikara, M. D., Dissanayake, D. R., Ishan, M., Sooriyapathirana, S. D. (2016). Dietar Anticancer and Medicinal Properties of the Phytochemicals in Chili Pepper (Capsicum spp.). Ceylon J Sci 45: 5–20
Oluwaniyi O., Oladipo J. (2017). Comparative Studies on the Phytochemicals, Nutrients and Antinutrients Content of Cassava Varieties. Journal of the Turkish Chemical Society Section A: Chemistry 4 (3): 661–674.
Mohammed AS A-MA. The effect of extract of the leaves of Adhatodav asicia plant against some types of bacteria contaminating the wounds by using an allergy test. J Umm Salamah of Sci. 2007;4(1):47-54.
Himratul-Aznita WH, Mohd-Al-Faisal N, Fathilah A. Determination of the percentage inhibition of diameter growth (PIDG) of Piper betle crude aqueous extract against oral Candida species. J Med Plant Res. 2011;5(6):878-84.
Korycka-Dahl M, Richardson T: Photogeneration of superoxide anion in serum of bovine milk and in model systems containing riboflavin and amino acids. J Dairy Sci. 1978, 61: 400-407.
Tylor BS, Kion YM, Wang QI, Sharpio RA, Billiar TR, Geller DA: Nitric oxide down regulates hepatocyte-inducible nitric oxide synthase gene expression. Arch Surg. 1997, 132: 1177-1183.
Miller MJ, Sadowska-Krowicka H, Chotinaruemol S, Kakkis JL, Clark DA: Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther. 1993, 264 (1): 11-16.
Balavoine GG, Geletti YV: Peroxynitrite scavenging by different antioxidants. Part 1: convenient study. Nitric oxide. 1999, 3: 40-54.
Prabakaran S., Ramu L., Veerappan, S., Pemiah B., Kannappan N. (2017). Effect of Different Solvents on Volatile and Non-Volatile Constituents of Red Bell Pepper (Capsicum annuum L.) and Their in vitro Antioxidant Activity. J Food Meas 11: 193–200.
Quettier-Deleu C., Gressier B., Vasseur J., Dine T., Brunet C., Luyckx M., Cazin M., Cazin J. C., Bailleul F., Trotin F.. (2000). Phenolic Compounds and Antioxidant Activities of Buckwheat. (Fagopyrum esculentum Moench) Hulls and Flour. J. Ethnopharmacol 72 (1-2): 35–42.
Adenike, A.O.O.; Nonye, I.I.; Olokayode, M.O. Characterization and recovery rates of foodindicator microorganisms from home-made oral rehydration solutions in Nigeria. African Journal of Biotechnology 2006, 5 (8), 603–608.
Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Breese, J.S.; Shapiro, C.; Giffin, P.M.; Tauxe, R.V. Food related illness and death in the United States. Emerging Infectious Diseases 1999, 5, 607–625.
National Committee for Clinical Laboratory Standards (NCCLS). Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. National Committee for Clinical Laboratory Standards: Wayne, PA, approved standard M7-A3, 1999.
N’guessan, J.D.; Coulibaly, A.; Ramanou, A.; Okou., O.C.; Djaman, A.J.; Guédé-Guina, F. Antibacterial activity of Thonningia Sanguinea against some multi-drug resistant strains of Salmonella enterica. African Health Sciences 2007, 7 (3), 155–158.
CCME 2 (7), 107-115 (2024) VISION PUBLISHER|116
Taylor, R.S.L.; Manandhar, N.P.; Towers, G.H.N. Screening of selected medicinal plants from Nepal for antimicrobial activities. Journal of Ethnopharmacology 1995, 46, 153–159.
Soberon, J.R.; Sgariglia, M.A.; Sampietro, D.A.; Quiroga, E.N.; Vattuone, M.A. Antibacterial activity of plant extracts from northwestern Argentina. Journal of Applied Microbiology 2006, 102, 1450–1461.
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Current Clinical and Medical Education













