Imaging Analysis Systems for Cell Classification, Fluorescence Microscopy Pictures, Medical Illness Diagnosis, and Environmental Monitoring Based on Cytometric Characteristics of Fluorescently Labelled Nuclei
Keywords:
Cytometric Features, Cell Classification, Fluorescently Labeled NucleiAbstract
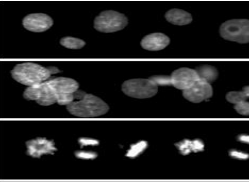
Classification of cells and tissues for clinical diagnostics and pharmaceutical and medical research relies heavily on nuclear features in human pathology and cytology. People have been trying to automate cytology analyses for decades in the hopes that it would make the process more efficient and the outcomes less subjective. Nuclear characteristics from fluorescently labelled images are priceless for image-based, high-content screening in drug discovery, functional genomics, cytomics, and diagnostic cell categorisation, among other applications. By collecting feature sets for every object encountered and using cell-by-cell data for categorisation, these screening systems describe the stimulus-induced behaviour of cell monolayer populations. Many of these applications rely on fluorescent nuclei detection to identify specific cells. This is due to several factors, including the abundance of easily accessible bright DNA dyes, the spatial separability of nuclei, and the abundance of extractable features related to artefact rejection, cytotoxicity, and cell cycle information. The automation of clinical diagnostic cytopathology has only reached mediocre progress despite enormous efforts. Cell classification performance, measuring the sample's cellular composition and disease progression, must be at least as good as that of human specialists, but the cost of fully automated diagnostic cytopathology instruments must be less than or equal to that of human analysis, for the instruments to be considered successful. To mimic the work of cytopathologists and cytotechnologists as nearly as possible, an ideal diagnostic tool would first accurately classify cells one by one, then compile a database of spatial relationships between cells, and lastly diagnose the entire lesion as well as individual cells. It is necessary to conduct a comprehensive evaluation of the cell nucleus in order to successfully classify millions of cells one by one and to identify cells in order to study their spatial relationships. In terms of diagnostic value, it is also crucial. This will serve as the focal point of our feature evaluation. Since fluorescence specificity produces the most precise picture segmentation and stoichiometry offers outstanding quantification, it is reasonable to presume that these features originated from pictures of fluorescently labelled cells.
Downloads
References
Bajaj S, Welsh JB, Leif RC, Price JH. Ultra-rare-event detection performance of a custom scanning cytometer on a model preparation of fetal nRBCs. Cytometry. 2000;39:285–294.
Kemp RA., MacAWay C., Garner D., Palcic B.: "Detection ofmalignancy associated changes in cervical cell nuclei using feed-forwath neural networks", Analytical Cellular Pathology 14: 31-40, 1997
Guillaud M., Doudkine A., Gamer D.: "Malignancy associated changes in cervical smeais: systematic changes in cytometiic features withthe grade of dysplasia", Analytical CellularPathology 9: 191-204, 1995
Steven F.S., Death M., Sin J., Palcic B.: "Fluorescent Location ofCells ofCytological Interest in Cervical Smears PreStained with Thionin", Anticancer Research 16: 1193-1196, 1996 21.
Wilbur D.C., Prey M.U., Miller W.M., Pawlick G.F., Colgan TJ.: 'Ilie AutoPap System for Primary Screening in Cervical Cytology," Acta Cytologica 42 (1): 214-220, 1998
BibbO M., Hawthorne C.: "Performance ofthe AutoPap Primaiy Screening System at Jefferson Univemity Hospital," Acta Cytologica 43 (1): 27-29, 1999
BibbO M., Hawthorne C., Zimmermann B.: Does the Use ofthe AutoPap Assisted Primary Screener Improve Cytologic Diagnosis?" Acta Cytologica 43 (1): 23-26, 1999
Ploem J.S., van Driel-Kulker A.MJ., Ploem-Zaaijer JJ.: "Automated Cell Analysis for DNA Studies of Large Cell Populations Using the LEYTAS Image Cytometry System," Pathology, Research and Practice 185 (5): 671-675, 1989
Baraldi A, Parmiggiani F. An investigation of the textural characteristics associated with gray level cooccurrence matrix statistical parameters. IEEE Transactions on Geoscience and Remote Sensing. 1995;33(2):293–304.
Bartels PH, Vooijs PG. Automation of primary screening for cervical cancer—sooner or later? Acta Cytologica. 1999;43(1):7–12.
Bartels PH, Thompson D, Weber JE. Diagnostic and prognostic decision support systems, Pathologic. 1995; 87(3):221–236.
Bibbo M, Hawthorne C. Performance of the AutoPap primary screening system at Jefferson University Hospital. Acta Cytologica. 1999;43(1):27–29.
Bibbo M, Hawthorne C, Zimmermann B. Does the use of the AutoPap assisted primary screener improve cytologic diagnosis? Acta Cytologica. 1999;43(1):23–26.
Birdsong GG. Automated screening of cervical cytology specimens. Human Pathology. 1996;27(5):468–481.
Boland MV, Murphy RF. A neural network classifier capable of recognizing the patterns of all major subcellular structures in fluorescence microscope images of HeLa cells. Bioinformatics. 2001;17(12):1213–1223.
Bravo-Zanoguera M, Laris CA, Nguyen LK, Oliva K, Price JH. Dynamic autofocus for continuousscanning time-delay-and-integration image acquisition in automated microscopy. Journal of Biomedical Optics. 2007;12(3):034011–034027.
Chen X, Zhou X,Wong ST. Automated segmentation, classification, and tracking of cancer cell nuclei in time-lapse microscopy. IEEE Transactions on Biomedical Engineering. 2006;53(4):762–766.
Chen C-C, Chen C-C. Filtering methods for texture discrimination. Pattern Recognition Letters. 1999;20:783–790.
Cortes C,Vapnik V. Support vector networks. Mach Learn. 1995;20:273–297. 13. Doudkine A, MacAulay C, Poulin N, Palcic B. Nuclear texture measurements in image cytometry. Pathologica. 1995;87(3):286–299.
Duda RO, Hart PE, Stork DG. Pattern Classification, 2nd ed. New York: John Wiley & Sons; 2001. 15. Duda RO, Hart PE. Pattern Classification and Scene Analysis. New York: John Wiley & Sons; 1973.
Flezar M, Doudkine A, Us-Krasovec M. The effect of primary fixation with standard postfixation and the duration of hydrolysis on nuclear features in image cytometry. Analytical Cellular Pathology. 1998;17: 131–144
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Current Clinical and Medical Education













