Chemical Characteristics of Nanoparticles: Cytotoxicity, Liposomal Modification, and Using of Nanoparticles in Cancer Therapy
Keywords:
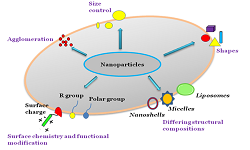
Nanoparticles, Chemical Characteristics, Modification, Cancer TherapyAbstract
There is no indication that the phenomenal growth of nanotechnology during the last four
decades will slow down. Nanotechnology and its ground breaking discoveries and products have
permeated every industry, from healthcare to food production. The use of nanoparticles has
considerably extended the shelf life of food items and enhanced the intracellular delivery of
hydrophobic drugs. A critical evaluation of the risks associated with nanoparticles utilised in
consumer items is urgently required due to the ever-increasing fascination with nanotechnology.
The general public views nanotechnology as having many beneficial impacts on human health,
which explains why this is the case. The composition is only one of several physicochemical
variables that influence a nanomaterial's toxicity; these properties also differ from those of bulk
materials. Size, area, chemistry, roughness, dispersion medium, and agglomeration ability are
some of these characteristics. As new nanoparticle-based products hit store shelves daily, there
is an urgent need to fill the knowledge gap on the relationship between physicochemical
properties and the emergence of toxicological issues. Despite the potential of targeted drug
delivery systems to improve cancer treatment, these methods are currently constrained by
tumour heterogeneity and micro-environmental challenges. Improving the delivery of targeted
therapies requires further study and innovation. Nanotechnology presents an exciting new
direction for cancer treatment by allowing for the targeted destruction of cancer cells with
minimal side effects on healthy tissues. The cancer microenvironment can be circumvented by
using nanoparticles to transport therapeutic medicines straight to the tumour site. Nanoparticles
can be made more effective by modifying their surfaces to increase their stability, circulation
time, and cellular absorption. Challenges including drug resistance and limited drug penetration
into solid tumours can be overcome with the use of nanoparticles in targeted therapy. To ensure
long-lasting and successful treatment, these nanoparticles can be designed to release the
therapeutic ingredients in a controlled way. In addition, the continuous progress in
nanotechnology has the ability to enable personalised medicine techniques that are adapted to
the specific demands of each patient, which might completely transform cancer treatment.
Downloads
References
M. Lin, L. Teng, Y. Wang, J. Zhang, X. Sun, Curcumin-guided nanotherapy: a lipidbased nanomedicine for targeted drug delivery in breast cancer therapy, Drug Deliv. 23 (2015) 1420–1425.
Ao, L. Huang, W. Su, Composite magnetic nanocarriers for MRI and targeted drug delivery, Nanomed. Nanotechnol. Biol. Med. 12 (2016) 490–491.
T.W. Wong, Use of microwave to improve nanomedicine application on skin, Expet Opin. Drug Deliv. 14 (2016), 283–283.
G. Sahay, NanoDDS, Engineering the next wave of nanomedicine and drug delivery systems, Adv. Drug Deliv. Rev. 144 (2019) (2018) 1–2.
S. Puri, M. Mazza, G. Roy, R.M. England, L. Zhou, S. Nourian, J. Anand Subramony, Evolution of nanomedicine formulations for targeted delivery and controlled release, Adv. Drug Deliv. Rev. (2023) 114962.
R. Müller, Junghanns, Nanocrystal Technology, Drug Delivery and Clinical Applications, Inter J of Nanomed, 2008, p. 295.
L.M. Bonanno, E. Segal, Nanostructured porous silicon–polymer-based hybrids: from biosensing to drug delivery, Nanomedicine (N. Y., NY, U. S.) 6 (2011) 1755–1770.
Y.-K. Oh, J. Huang, Editorial: graphene-based materials in nanomedicine, Adv. Drug Deliv. Rev. 105 (2016) 107–108.
E. Fattal, F. Fay, Nanomedicine-based delivery strategies for nucleic acid gene inhibitors in inflammatory diseases, Adv. Drug Deliv. Rev. 175 (2021) 113809.
CCME 2 (8), 319-336 (2024) VISION PUBLISHER|335
J. Ding, X. Chen, Functional polymer nanocarriers for rational antitumor drug delivery, Nanomed. Nanotechnol. Biol. Med. 14 (2018) 1875–1876.
E. Nance, Careers in nanomedicine and drug delivery, Adv. Drug Deliv. Rev. 144 (2019) 180–189.
Y. Sun, C. Kang, F. Liu, Y. Zhou, L. Luo, H. Qiao, RGD peptide-based target drug delivery of doxorubicin nanomedicine, Drug Dev. Res. 78 (2017) 283–291.
J. Dobson, Magnetic micro- and nano-particle-based targeting for drug and gene delivery, Nanomedicine (N. Y., NY, U. S.) 1 (2006) 31–37.
Q. Liu, J. Du, Asymmetrical polymer vesicles for significantly improving MRI sensitivity and cancer-targeted drug delivery, Nanomed. Nanotechnol. Biol. Med. 12 (2016) 483.
M. Swierczewska, H.S. Han, K. Kim, J.H. Park, S. Lee, Polysaccharide-based nanoparticles for theranostic nanomedicine, Adv. Drug Deliv. Rev. 99 (2016) 70–84.
T.M. Nguyen, S. Lee, S.B. Lee, Conductive polymer nanotube patch for fast and controlled ex vivo transdermal drug delivery, Nanomedicine (N. Y., NY, U. S.) 9 (2014) 2263–2272.
F. Shakeel, Editorial: nanomedicine-based drug delivery systems: recent developments and future prospects, Molecules 28 (2023) 4138.
J. Ouyang, S. Rao, R. Liu, L. Wang, W. Chen, W. Tao, N. Kong, 2D materials-based nanomedicine: from discovery to applications, Adv. Drug Deliv. Rev. 185 (2022) 114268.
M. Michalak, P. Kurcok, M. Hakkarainen, Polyhydroxyalkanoate-based drug delivery systems, Pol. Internet 66 (2016) 617–622.
D.A. Hafez, K.A. Elkhodairy, M. Teleb, A.O. Elzoghby, Nanomedicine-based approaches for improved delivery of phyto-therapeutics for cancer therapy, Expet Opin. Drug Deliv. 17 (2020) 279–285.
Z. Chen, X. Lai, S. Song, X. Zhu, J. Zhu, Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy, Drug Deliv. 23 (2015) 1369–1373.
M.K. Viswanadh, M.S. Muthu, Targeted bioadhesive nanomedicine: an effective approach for synergistic drug delivery to cancers, Nanomedicine (N. Y., NY, U. S.) 13 (2018) 1401–1403.
M.K. Viswanadh, M.S. Muthu, Targeted bioadhesive nanomedicine: an effective approach for synergistic drug delivery to cancers, Nanomedicine (N. Y., NY, U. S.) 13 (2018) 1401–1403.
Z. Chen, X. Lai, S. Song, X. Zhu, J. Zhu, Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy, Drug Deliv. 23 (2015) 1369–1373.
P.J. Harrison, H. Wieslander, A. Sabirsh, J. Karlsson, V. Malmsjo, A. Hellander, € C. W€ahlby, O. Spjuth, Deep-learning models for lipid nanoparticle-based drug delivery, Nanomedicine (N. Y., NY, U. S.) 16 (2021) 1097–1110.
J. Li, L. Sun, Y. Liu, H. Yao, S. Jiang, YunzhuPu, Y. Li, Y. Zhang, To reduce premature drug release while ensuring burst intracellular drug release of solid lipid nanoparticle-based drug delivery system with clathrin modification, Nanomed. Nanotechnol. Biol. Med. 15 (2019) 108–118.
S. Kotta, N. Sharma, P. Raturi, M. Aleem, R.K. Sharma, Exploring novel strategies for lipid-based drug delivery, J of Nano and Nanomed 3 (2018) 1–22.
J. Dobson, Magnetic micro- and nano-particle-based targeting for drug and gene delivery, Nanomedicine (N. Y., NY, U. S.) 1 (2006) 31–37.
E. Nance, Careers in nanomedicine and drug delivery, Adv. Drug Deliv. Rev. 144 (2019) 180–189.
CCME 2 (8), 319-336 (2024) VISION PUBLISHER|336
C.R. Garcia, A.T. Rad, F. Saeedinejad, A. Manojkumar, D. Roy, H. Rodrigo, S.A. Chew, Z. Rahman, M.-P. Nieh, U. Roy, Effect of drug-to-lipid ratio on nanodisc-based tenofovir drug delivery to the brain for HIV-1 infection, Nanomedicine (N. Y., NY, U. S.) 17 (2022) 959–978.
C.V. Kulkarni, Applications of lipid nanostructures and hybrid systems for drug delivery, J. Nanomed. Nanotechnol. 07 (2016) 58.
P. Jaiswal, B. Gidwani, A. Vyas, Nanostructured lipid carriers and their current application in targeted drug delivery Artificial Cells, Nanomed Biotech 44 (2014) 27–40.
W. Gao, Y. Chen, Y. Zhang, Q. Zhang, L. Zhang, Nanoparticle-based local antimicrobial drug delivery, Adv. Drug Deliv. Rev. 127 (2018) 46–57.
D. Zhu, W. Tao, H. Zhang, G. Liu, T. Wang, L. Zhang, X. Zeng, L. Mei, Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer, Acta Biomater. 30 (2016) 144–154,
J. Hu, S. Fu, Q. Peng, Y. Han, J. Xie, N. Zan, Y. Chen, J. Fan, Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation, Int. J. Pharm. 516 (2017) 313–322,
M. Jin, G. Jin, L. Kang, L. Chen, Z. Gao, W. Huang, Smart polymeric nanoparticles with pH-responsive and PEG-detachable properties for co-delivering paclitaxel and survivin siRNA to enhance antitumor outcomes, Int. J. Nanomed. 13 (2018) 2405–2426,
Y. Çırpanlı, E. Allard, C. Passirani, E. Bilensoy, L. Lemaire, S. Çalıs¸, J.-P. Benoit, Antitumoral activity of camptothecin-loaded nanoparticles in 9L rat glioma model, Int. J. Pharm. 403 (2011) 201–206,
Guo, X. Gao, L. Su, H. Xia, G. Gu, Z. Pang, X. Jiang, L. Yao, J. Chen, H. Chen, Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery, Biomaterials 32 (2011) 8010, https://doi.org/10.1016/ j.biomaterials.2011.07.004. –8020. [65] Y. Zhao, C. Cai, M. Liu, Y. Zhao, W. Pei, X. Chu, H. Zhang, Z. Wang, J. Han, An organic solvent-free technology for the fabrication of albumin-based paclitaxel nanoparticles for effective cancer therapy, Colloids Surf. B Biointerfaces 183 (2019) 110394,
T. Hekmatara, C. Bernreuther, A.S. Khalansky, A. Theisen, J. Weissenberger, J. Matschke, S. Gelperina, J. Kreuter, M. Glatzel, Efficient systemic therapy of rat glioblastoma by nanoparticle-bound doxorubicin is due to antiangiogenic effects, Clin. Neuropathol. 28 (2009) 153–164,
Y. Malinovskaya, P. Melnikov, V. Baklaushev, A. Gabashvili, N. Osipova, S. Mantrov, Y. Ermolenko, O. Maksimenko, M. Gorshkova, V. Balabanyan, J. Kreuter, S. Gelperina, Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells, Int. J. Pharm. 524 (2017) 77–90,
V. Khanna, S. Kalscheuer, A. Kirtane, W. Zhang, J. Panyam, Perlecan-targeted nanoparticles for drug delivery to triple-negative breast cancer, Future Drug Discovery 1 (2019).
A. Patil, P. Lakhani, P. Taskar, K.-W. Wu, C. Sweeney, B. Avula, Y.-H. Wang, I.A. Khan, S. Majumdar, Formulation development, optimization, and in vitro–in vivo characterization of natamycin-loaded PEGylated nano-lipid carriers for ocular applications, J. Pharmaceut. Sci. 107 (2018) 2160–2171,
G.K. Zorzi, R.S. Schuh, V.J. Maschio, N.T. Brazil, M.B. Rott, H.F. Teixeira, Box Behnken design of siRNA-loaded liposomes for the treatment of a murine model of ocular keratitis caused by Acanthamoeba, Colloids Surf. B Biointerfaces 173 (2019) 725–732,
P. Jansook, P. Kulsirachote, R. Asasutjarit, T. Loftsson, Development of celecoxib eye drop solution and microsuspension: a comparative investigation of binary and ternary cyclodextrin complexes, Carbohydr. Polym. 225 (2019) 115209,
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Awab Akab Hawar Abd AL-Akashi, Rawan Fares Theab Ahmed Al – Samarraie, Sorour Khalil Mohsen khinjar alzadi, Lara ali abbas hamad aldaraji

This work is licensed under a Creative Commons Attribution 4.0 International License.
Current Clinical and Medical Education













