Metabolic Effects of Dietary Fiber, Carbohydrate, Glucose, Lipid Metabolism on Human Health and Nutritional Therapy
Keywords:
Nutritional Therapy, Metabolic, Dietary Fiber, MetabolismAbstract
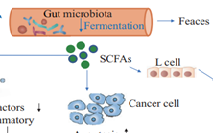
The majority of diabetic and nutrition groups recommend a diet rich in dietary fibre (DF). Soluble DF is known to have favourable effects on certain blood lipids, decrease the postprandial glucose response, and restrict macronutrient absorption due to its viscous and gel-forming characteristics. While there appears to be no discernible difference in the regulation of body weight between soluble and insoluble DF consumption, the fermentation of naturally occurring high-fiber foods in the colon is mostly caused by soluble DF. Nevertheless, prospective cohort studies have consistently linked insoluble cereal DF and whole grains—rather than soluble DF—to a lower risk of diabetes, indicating that additional, as-yet-unidentified mechanisms are probably at work. New evidence suggests that DF consumption does more than just affect weight. It also improves insulin sensitivity, modifies the secretion of gut hormones, and affects metabolic and inflammatory markers linked to metabolic syndrome, among other surprising metabolic effects. A common dietary strategy for improving hyperglycemia and diabetes is increasing consumption of dietary fibre, which consists of indigestible polysaccharides. Dietary fibres like guar, inulin, cellulose, and pectin have been shown to regulate glucose metabolism, according to numerous studies. Another new dietary fibre that reduces postprandial glycemia is an indigestible polysaccharide. A lot of people are curious about how fibre affects carbohydrate metabolism because someone said that diabetes should be one of many Western diseases linked to not getting enough fibre. 1 Research on fiber's effects on diabetes has only served to fuel interest in this field. Researchers Anderson and Ward3 and Kiehm et al.2 discovered that low-insulin diabetes patients' insulin needs were alleviated when they followed high-carbohydrate, high-fiber diets. No matter the insulin dosage, Jenkins et al.4,5 found that diabetics' glycosuria decreased when guar gum was added to their diet. Miranda and Horwitz6 found that diabetics whose breads were high in cellulose had a more consistent glucose profile throughout the day. Developing medications for diabetics that aim to change small-intestinal events became possible as a result of these investigations. They also offered a chance to study and improve the overall glucose metabolism that came from these alterations. Consequently, research into the physicochemical features that may influence glucose tolerance and the kinds of dietary fibre that may have the greatest impact on this area has been accelerated.
Downloads
References
Jenkins DJ, Wolever TM, Leeds AR, Gassull MA, Haisman P, et al. (1978) Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. Br Med J 1(6124): 1392-1394.
Johnson IT, Gee JM (1981) Effect of gel-forming gums on the intestinal unstirred layer and sugar transport in vitro. Gut 22(5): 398-403.
Kaur N, Gupta AK (2002) Applications of inulin and oligofructose in health and nutrition. J Biosci 27(7): 703-714.
Kay RM (1982) Dietary fiber. J Lipid Res 23(2):221-242.
Flourie B, Vidon N, Florent CH, Bernier JJ (1984) Effect of pectin on jejunal glucose absorption and unstirred layer thickness in normal man. Gut 25(9): 936-941.
Hills RD Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, et al. (2019) Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 11(7): 1613.
Beam A, Clinger E, Hao L (2021) Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 13(8): 2795.
Birkeland E, Gharagozlian S, Birkeland KI, Valeur J, Måge I, et al. (2020) Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: a randomised controlled trial. Eur J Nutr 59(7): 3325-3338.
Shao T, Yu Q, Zhu T, Liu A, Gao X, et al. (2020) Inulin from Jerusalem artichoke tubers alleviates hyperglycaemia in high-fat-diet-induced diabetes mice through the intestinal microflora improvement. Br J Nutr 123(3): 308-318.
Sadakiyo T, Ishida Y, Inoue SI, Taniguchi Y, Sakurai T, et al. (2017) Attenuation of postprandial blood glucose in humans consuming isomaltodextrin: carbohydrate loading studies. Food Nutr Res 61(1): 1325306.
Tsuchiya Y, Kawamata K, Tomita M, Tsuboi M, Takahashi T, et al. (2015) Effects of Salmon Nasal Cartilage Proteoglycan on Plasma Glucose Concentration and Active Glucose Transport in the Small Intestine. J Nutr Sci Vitaminol (Tokyo) 61(6): 502-505.
Majima M, Takagaki K, Sudo S, Yoshihara S, Kudo Y, et al. (2001) Effect of proteoglycan on experimental colitis. Int Congr Ser 1223: 221-224.
Sashinami H, Takagaki K, Nakane A (2006) Salmon cartilage proteoglycan modulates cytokine responses to Escherichia coli in mouse macrophages. BiochemBiophys Res Commun 351(4): 1005-1010.
M. Elleuch, D. Bedigian, O. Roiseux, et al., Dietary fibre and fibre-rich byproducts of food processing: characterisation, technological functionality and commercial applications: a review, Food Chem. 124(2) (2011) 411-421.
M.J. Gidley, G.E. Yakubov, Functional categorisation of dietary fibre in foods: beyond ‘soluble’ vs ‘insoluble’, Trends Food Sci. Tech. 86 (2019) 563-568.
A. Olson, G.M. Gray, M.C. Chiu, et al., Chemistry and analysis of soluble dietary fiber, Food Tech. 41(2) (1987) 71-80
M.A. Eastwood, The physiological effects of dietary fiber: an update, Annu. Rev. Nutr. 12 (1992) 19-35.
J.M. Lattimer, M.D. Haub, Effects of dietary fiber and its components on metabolic health, Nutrients 2(12) (2010) 1266-1289.
W.G.T. Willats, J.P. Knox, J.D. Mikkelsen, Pectin: new insights into an old polymer are starting to gel, Trends Food Sci. Tech. 17(3) (2006) 97-104.
J.L. Doublier, P.J. Wood, Rheological properties of aqueous solutions of (1,3) (1,4)-beta-D-glucan from oats (Avena sativa L.), Cereal Chem. 72(4) (1995) 335-340.
K.M. Davison, N.J. Temple, Cereal fiber, fruit fiber, and type 2 diabetes: explaining the paradox, J. Diabetes Complicat. 32(2) (2018) 240-245.
A.H. Hite, Nutritional epidemiology of chronic disease and defining “Healthy Diet”, Glob. Food Sec. 4(2) (2018) 207-225.
D.S. Ludwig, F.B. Hu, L. Tappy, et al., Dietary carbohydrates: role of quality and quantity in chronic disease, BMJ 361 (2018) 2340.
J. Yu, M. Ahmedna, Functional components of grape pomace: their composition, biological properties and potential applications, Int. J. Food Sci. Tech. 48(2) (2013) 221-237.
T.H. Zhang, Y. Yang, Y. Liang, et al., Beneficial effect of intestinal fermentation of natural polysaccharides, Nutrients 10(8) (2018) 1055.
B. Wei, Y. Liu, X. Lin, et al., Dietary fiber intake and risk of metabolic syndrome: a meta-analysis of observational studies, Clin. Nutr. 37(6) (2018) 1935-1942.
J.A. Robertson, F.D.D. Monredon, P. Dysseler, et al., Hydration properties of dietary fibre and resistant starch: a European collaborative study, LWT-Food Sci. Technol. 33(2) (2000) 72-79.
F. Xie, Y. Wang, J. Wu, et al., Insoluble dietary fibers from Angelica keiskeiby-product and their functional and morphological properties, Starch-Stärke 68 (2016) 1-12.
Y. Zheng, Y. Li, Physicochemical and functional properties of coconut (Cocos nucifera L) cake dietary fibers: effects of cellulase hydrolysis, acid treatment and particle size distribution, Food Chem. 257(15) (2018) 135-142.
H.C. Chao, M.W. Lai, M.S. Kong, et al. Cutoff volume of dietary fiber to ameliorate constipation in children, J. Pediatr. 153(1) (2008) 45-49.
J.E. Lambert, J.A. Parnell, J.M. Tunnicliffe, et al., Consuming yellow pea fiber reduces voluntary energy intake and body fat in overweight/obese adults in a 12-week randomized controlled trial, Clin. Nutr. 36 (2017) 126-133.
W.J. Dahl, M.L. Stewart, Position of the academy of nutrition and dietetics: health implications of dietary fiber, J. Acad. Nutr. Diet. 115(11) (2015) 1861-1870.
S. Morozov, V. Isakov, M. Konovalova, Fiber-enriched diet helps to control symptoms and improves esophageal motility in patients with nonerosive gastroesophageal reflux disease, World J. Gastroentero. 24 (2018) 2291-2299.
X. Zhou, L. Zhang, W.J. Ji, et al., Variation in dietary salt intake induces coordinated dynamics of monocyte subsets and monocyte-platelet aggregates in humans: implications in end organ inflammation, Plos One 8(4) (2013) 60332.
L. Kuniak, R.H. Marchessault, Study of the crosslinking reaction between epichlorohydrin and starch, Starch-StÃrke. 24 (2010) 110-116.
F. Xie, M. Li, X. Lan, et al., Modification of dietary fibers from purplefleshed potatoes (Heimeiren) with high hydrostatic pressure and high pressure homogenization processing: a comparative study, Innov. Food Sci. Emerg. 42 (2017) 157-164.
H. Chen, C. Zhao, J. Li, et al., Effects of extrusion on structural and physicochemical properties of soluble dietary fiber from nodes of lotus root, LWT-Food Sci. Technol. 93 (2018) 204-211.
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Current Clinical and Medical Education













