ASSOCIATION OF GENETICALLY MODIFIED HERPES VIRUS IN THE TREATMENT OF MELANOMAS
Keywords:
Oncolytic Viruses ; T-VEC; Talimogene Laherparepvec ; HSV-1; MelanomaAbstract
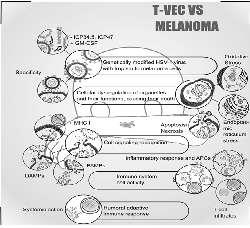
Introduction: Melanoma skin cancer is the most aggressive and lethal due to its ability to develop metastases. Considering that conventional treatments do not provide desired results, the search for new methods is crucial. Thus, oncolytic viruses, specifically Talimogene Laherparepvec (T-VEC), which is a genetically modified herpes simplex virus (HSV-1), have been introduced as an effective treatment for non-resectable melanoma. T-VEC acts on tumor cells through viral replication and induction of the immune system. This article aims to discuss the use of T-VEC in melanoma treatment, its effects, and results, in order to shed light on its mechanism of action. Methods: A qualitative and exploratory review was conducted by searching scientific articles in PubMed and ScienceDirect. The articles were selected based on relevance criteria, including theme, approach to the subject, and results. Only articles published between 2020 and 2022 were considered. Results: The literature reveals that treatments with T-VEC have shown remarkable results in terms of metastasis regression and overcoming resistance to immunotherapy. Combined therapies have demonstrated even greater relevance compared to monotherapy. T-VEC used as a first-line therapy has shown longer progression-free survival. Conclusion: The use of Talimogene Laherparepvec (T-VEC) therapy demonstrates its potential to combat melanoma, despite the presence of some adverse effects during the treatment process. It remains one of the most promising tools for effective intervention in melanoma.
Downloads
References
Bayan CY, Lopez AT, Gartrell RD, et al. The Role of Oncolytic Viruses in the Treatment of Melanoma [published correction appears in Curr Oncol Rep. 2018 Nov 13;20(12):100]. Curr Oncol Rep. 2018;20(10):80. Published 2018 Aug 25. doi:10.1007/s11912-018-0729-3
BRASIL. Instituto Nacional de Câncer. Ministério da Saúde (org.). Estimativa 2020. 2021. https://www.inca.gov.br/estimativa/taxas-ajustadas/melanoma-maligno-da-pele
Brennan P, Davey-Smith G. Identifying Novel Causes of Cancers to Enhance Cancer Prevention: New Strategies Are Needed. J Natl Cancer Inst. 2022;114(3):353-360. doi:10.1093/jnci/djab204
MOURA PF, PAULA CDS, OLIVEIRA CF de, MIGUEL MD. Câncer de pele: Uma questão de saúde pública. Visão Acadêmica. 2017 Mar 24;17(4).
WHO. World Health Organization. Radiation: Ultraviolet (UV) radiation and skin cancer. 2017. https://www.who.int/news-room/questions-and-answers/item/radiation-ultraviolet-(uv)-radiation-and-skin-cancer
Reale A, Vitiello A, Conciatori V, Parolin C, Calistri A, Palù G. Perspectives on immunotherapy via oncolytic viruses. Infect Agent Cancer. 2019;14:5. Published 2019 Feb 11. doi:10.1186/s13027-018-0218-1
Corrigan PA, Beaulieu C, Patel RB, Lowe DK. Talimogene Laherparepvec: An Oncolytic Virus Therapy for Melanoma. Ann Pharmacother. 2017;51(8):675-681. doi:10.1177/1060028017702654
Fu LQ, Wang SB, Cai MH, et al. Recent advances in oncolytic virus-based cancer therapy. Virus Res. 2019;270:197675. doi:10.1016/j.virusres.2019.197675
Santos Apolonio J, Lima de Souza Gonçalves V, Cordeiro Santos ML, et al. Oncolytic virus therapy in cancer: A current review. World J Virol. 2021;10(5):229-255. doi:10.5501/wjv.v10.i5.229
BRASIL. Ministério da Saúde. Instituto Nacional do Câncer. Estimativa 2020 - Síntese de Resultados e Comentários: Câncer de pele. [S. l.]: Instituto Nacional do Câncer, 12 may 2020. https://www.inca.gov.br/estimativa/sintese-de-resultados-e-comentarios
BRASIL. Ministério da Saúde. Instituto Nacional do Câncer. Tratamento do câncer: Radioterapia. [S. l.]: Instituto Nacional do Câncer, 2021. https://www.inca.gov.br/tratamento/radioterapia.
Fröhlich A, Niebel D, Fietz S, et al. Talimogene laherparepvec treatment to overcome loco-regional acquired resistance to immune checkpoint blockade in tumor stage IIIB-IV M1c melanoma patients. Cancer Immunol Immunother. 2020;69(5):759-769. doi:10.1007/s00262-020-02487-x
Louie KS, Banks V, Scholz F, et al. Real-world use of talimogene laherparepvec in Germany: a retrospective observational study using a prescription database. Future Oncol. 2020;16(8):317-328. doi:10.2217/fon-2019-0838
Bhatt DK, Wekema L, Carvalho Barros LR, Chammas R, Daemen T. A systematic analysis on the clinical safety and efficacy of onco-virotherapy. Molecular Therapy Oncolytics. 2021;23:239-253. doi:10.1016/j.omto.2021.09.008
Malvehy J, Samoylenko I, Schadendorf D, et al. Talimogene laherparepvec upregulates immune-cell populations in non-injected lesions: findings from a phase II, multicenter, open-label study in patients with stage IIIB-IVM1c melanoma. J Immunother Cancer. 2021;9(3):e001621. doi:10.1136/jitc-2020-001621
Ressler JM, Karasek M, Koch L, et al. Real-life use of talimogene laherparepvec (T-VEC) in melanoma patients in centers in Austria, Switzerland and Germany [published correction appears in J Immunother Cancer. 2021 Oct;9(10):]. J Immunother Cancer. 2021;9(2):e001701. doi:10.1136/jitc-2020-001701
van der Leun AM, Haanen JB. The power to "di-sc-seq-t". Cancer Cell. 2021;39(3):299-301. doi:10.1016/j.ccell.2021.02.001
Kalus P, De Munck J, Vanbellingen S, et al. Oncolytic Herpes Simplex Virus Type 1 Induces Immunogenic Cell Death Resulting in Maturation of BDCA-1+ Myeloid Dendritic Cells. Int J Mol Sci. 2022;23(9):4865. Published 2022 Apr 27. doi:10.3390/ijms23094865
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Filipe Vicente Muricy, Mirella Paiva Solidade, Daniel Pereira Passos, Marisa Salvi, Marcos Vinicius Cardoso Matos Silva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Current Clinical and Medical Education













