Preparation and Characterization of Chitosan Nanoparticles from Crab shell and Determination their Activity as Antibacterial and Antioxidant
Keywords:
CS-NPs, Crab shell, Anti-bacterial, antioxidantAbstract
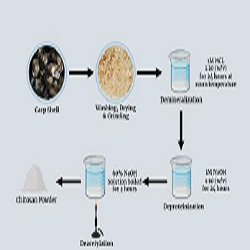
Back ground: Chitosan nanoparticles (CS-NPs) are potential materials for medical application like antibacterial, antioxidant and anticancer. Objective: This study was focused on preparation of CS-NPs from wasted crab shell, determination their antibacterial and antioxidant activity. Methodology: The CS-NPs were prepared by using the ionic gelation method, characterization of CS-NPs includes Scanning Electron Microscopy (SEM) and X-ray Diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR Analysis) and determination their antibacterial against (E. coli and Streptococcus mutanse) and antioxident activity by DPPH test. Results: crystalline nature of the prepared nanoparticles with average size around 32.2 nm scale with rod shape. The synthesized CS-NPs showed antibacterial efficacy against clinical bacterial isolates of MDR in both Gram positive, Streptococcus mutanse, and Gram negative, E. coli By using the disk diffusion method. This activity might be attributed to the unique biological and physicochemical properties of the CS-NPs, which facilitate the disruption of bacterial cell membranes, from results underscore the potential of crab shellas an eco-friendly and efficient biological agent for the synthesis of CS-NPs with potent antibacterial properties. The prepared CS-NPs revealed a significant high antioxidant activity compared to ascorbic acid in the same concentration. Conclusion: It can be concluded that CS-NPs can be prepared from carb sell in an eco-friendly way and this CS-NPs can used as a cost-effective antibacterial agent and antioxidant.
Downloads
References
Nitta, S.K., Numata, K., Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering, Int. J. Mol. Sci, 14 (2013) 1629-1654
Kataoka, K., Harada, A., Nagasaki, Y., Block copolymer micelles for drug delivery: Design, characterization and biological significance, Adv. Drug Deliv. Rev., 64(2012) 37-48.
Panyam, J., Labhasetwar, V., Biodegradable nanoparticles for drug and gene delivery to cells and tissue, Adv. Drug Deliv. Rev., 55(2003) 329-347.
Shi, J., Votruba, A.R., Farokhzad, O.C., Langer, R., Nanotechnology in drug delivery and tissue engineering: From discovery to applications, Nano letters, 10(2010) 3223-3230.
Joye, I.J., McClements, D.J., Emulsifying and emulsionstabilizing properties of gluten hydrolysates, J. Agric. Food Chem., 62(2014) 2623-2630.
A.M. Omer, R. Dey, A.S. Eltaweil, E.M. Abd El- Monaem, and Z. M. Ziora, Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions, Arabian Journal Chemistry, 15(2) (2022) 103543.
W. Liu, J. Xie, L. Li, Properties of phenolic acid- chitosan composite films and preservative effect on Penaeus vannamei, Journal Molecular Structure, 1239(1) (2021) 130531.
S. Chen, Z. Zhang, X. Wei, Z. Sui, J. Geng, Antibacterial and antioxidant water-degradable food packaging chitosan film prepared from American cockroach, Food Bioscience, 49(2) (2022) 101893.
Y. Cao, S. Alamri, A. A. Rajhi, A. E. Anqi, and A. D. Khalaji, New chitosan Schiff base and its nanocomposite: Removal of methyl green from aqueous solution and its antibacterial activities, International Journal Biology Macromolecule, 192 (2021) 1–6.
B. Tian, Y. Liu, and J. Liu, Chitosan-based nanoscale and non-nanoscale delivery systems for anticancer drugs: A review, European Polymer Journal, 154 (2021) 110533.
M. Hosny, M. Fawzy, E. El-Fakhrany, A. Omer, E. El-Monaem, R. Khalifa, and A. Eltaweil, Biogenic synthesis, characterization, antimicrobial, antioxidant, antidiabetic, and catalytic applications of platinum nanoparticles synthesized from Polygonum salicifolium leaves, Journal Environmental Chemical Engineering, 10(1) (2022) 106806.
S. Chatterjee, A.K. Guha, and B.P. Chatterjee, Evaluation of quantity and quality of chitosan produce from Rhizopus oryzae by utilizing food product processing waste whey and molasses, Journal Environmental Management, 251 (2019) 109565 #
Calvo, P., Remunan-Lopez, C., Vila-Jato, J.L., Alonso, M.J., Novel hydrophilic chitosan–polyethylene oxide nanoparticles as protein carriers, J. Appl. Polym. Sci., 63(1997) 125-132.
Kumar, V., Dandapat, S., Kumar, A., Kumar, N., Preparation and characterization of chitosan nanoparticles “alternatively, carrying potential” for cellular and humoral immune responses, Adv. Anim. Vet. Sci., 2 (2014) 414-417.
Samrot, A.V., Jahnavi, T., Padmanaban, S., Philip, S.A,. Burman, U., Rabel, A.M., Chelators influenced synthesis of chitosan–carboxymethyl cellulose microparticles for controlled drug delivery, Appl. Nanosci., 6(2016) 1219-1231.
Amidi, M., Mastrobattista, E., Jiskoot, W., Hennink, W.E,. Chitosan-based delivery systems for protein therapeutics and antigens, Adv. Drug Deliv. Rev., 62(2010) 59-82.
C. Moon, D.J. Seo, Y.S. Song, and W.J. Jung, Antibacterial activity of various chitosan forms against Xanthomonas axonopodis pv. glycines, Elsevier B.V, 156 (2020)
A.M.L. Kusnadi, Purgiyanti, A.C. Kumoro, The antioxidant and antibacterial activity of chitosan extract from white shrimp shell (Penaueus indicus) in the water nortyh of Brebes, Indonesia, Biodiversity, 23(3) (2022) 1267– 1272.
M. Chandrasekaran, K.D. Kim, and S.C. Chun, Antibacterial activity of chitosan nanoparticles: A review, Processes, 8(9) (2020), 1–21.
A.S. Kritchenkov, M.N. Kurasova, A.A. Godzishevskaya, E.S. Mitrofanova, High antibacterial activity and low toxicity of pyridoxal derivatives of chitosan and their nanoparticles, Mendeleev Communication, 31(4) (2021) 504–506.
D. MubarakAli, F.L. Oscar, V. Gopinath, N.S. Alharbi, S.A. Alharbi, and N. Thajuddin, An inhibitory action of chitosan nanoparticles against pathogenic bacteria and fungi and their potential applications as biocompatible antioxidants, Microbiology Pathogenesis, 114(2) (2018) 323–327.
Samar, M.M.; El-Kalyoubi, M.; Khalaf, M.; El-Razik, M.A. Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann. Agric. Sci. 2013, 58, 33–41.
Hafsa, J.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Ann. Pharm. Françaises 2016, 74, 27–33.
Prabu, K.; Natarajan, E. In Vitro Antimicrobial and Antioxidant Activity of Chitosan Isolated from Podophthalmus vigil. J. Appl. Pharm. Sci. 2012, 9, 75–82.
CLSI. Clinical and Laboratory Standards Institute (CLSI). 2020. Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI Suppl. M100.
Puvvada et al., 2012
Sumaila A., Ndamitso M. M., Iyaka Y.A., Abdulkareem, A.S., Tijani J. O., Idris M.O.(2020). Extraction and Characterization of Chitosan from Crab Shells: Kinetic and Thermodynamic Studies of Arsenic and Copper Adsorption from Electroplating Wastewater. Iraqi Journal of Science, Vol. 61, No. 9, pp: 2156-2171 .
Olán, G.M.; Ríos, M.A.; Selene, A.; Cázares, C.H.; Morán, P.Z.; Suárez, S.L.; -López, M.R.(2022). Effect of Chitosan Nanoparticles Incorporating Antioxidants from Salvia hispanica L. on the Amaranth Flour Films. Food Technol. Biotechnol. 60 (1) 52–66. original scientific paper ISSN 1330-9862.
Jaworska M, Kula K, Chassary P, Guibal E (2003) Influence of chitosan characteristics on polymer properties: II. Platinum sorption properties. Polymer International 52 (2): 206–212.
Maram THAK, Mohammed R, Maher ZE (2013). Wastewater treatment with chitosan nano-particles. International Journal of Nanotechnology and Application 3 (2): 39-50.
Olajide A, Okoronkwo AE, Oluwasina OO, Abe TO (2018) [14] Utilization of blue crab shells for the synthesis of CHNP and their characterization. Songklanakarin J Sci Technol 40 (5): 1043-1047.
Maram THAK, Mohammed R, Maher ZE (2013) Wastewater treatment with chitosan nano-particles. International Journal of Nanotechnology and Application 3 (2): 39-50..
Jaworska M, Kula K, Chassary P, Guibal E (2003). Influence of chitosan characteristics on polymer properties: II. Platinum sorption properties. Polymer International 52 (2): 206–212.
Momin, B.; Rahman, S.; Jha, N. and Annapure, U. S. (2019). Valorization of mutant Bacillus licheniformis M09 supernatant for green synthesis of silver nanoparticles: photocatalytic dye degradation, antibacterial activity, and cytotoxicity. Bioprocess and biosystems engineering, 42(4): 541-553.
Vijayakumar, S.; Malaikozhundan, B.; Parthasarathy, A.; Saravanakumar, K.; Wang, M. H. and Vaseeharan, B. (2020). Nano biomedical potential of biopolymer chitosan-capped silver nanoparticles with special reference to antibacterial, antibiofilm, anticoagulant and wound dressing material. Journal of Cluster Science, 31(2): 355-366.
Sari L. R.; Sumpono and Elvinawati. (2019). Uji efektifitas asap cair cangkang buah karet (Hevea braziliensis) sebagai antibakteri Bacillus subtilis. Jurnal Pendidikan dan Ilmu Kimia, 3(1):34-40.
Sumampoue O. J. (2018). Uji sensitivitas antibiotik terhadap bakteri Escherichia coli penyebab diare balita di Kota Manado. Journal of Current Pharmaceutical Sciences, 2(1):104-110.
S. Magaldia; S. Mata-Essayaga; C. Hartung de Caprilesa; C. Pereza; M.T. Colella a; Carolina Olaizolaa and Yudith Ontiverosb. (2004). Well diffusion for antifungal susceptibility testing. International Journal of Infectious Diseasesm 8:39-45.
Sarwono R. (2010). Pemanfaatan kitin/kitosan sebagai bahan anti mikroba. Jurnal Kimia Terapan Indonesia, 12(1):32-38.
Yamaguchi T., Takamura H., Matoba T., Terao J. (1998).HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1,-diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998;62:1201–1204. doi: 10.1271/bbb.62.1201.
Abdulazeem , L., Mohammad J. AL Jassani, Mustafa A. Al-Sheakh. (2021). Free Radical Scavenging and Antioxidant Activity of Silver Nanoparticles Synthesized from Cuminum cyminum (Cumin) seed Extract. Research J. Pharm. and Tech. 14(8):pp;1-6.
Wang, W.; Chen, Q.; Jiang, C.; Yang, D.; Liu, X.and Xu, S. One-step synthesis of biocompatible gold nanoparticles using gallic acid in the presence of poly-(Nvinyl-pyrrolidone). Colloids Surf A Physicochem Eng Asp. 2007, 301,P:73-9 .
Awika, J.M..; Rooney, L.W.; Wu, X.; Prior, R.L. and Zevallos, L.C. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J Agric Food Chem.; 2003, 51, P:6657-6662 https://doi.org/10.1016/j.jes.2018.05.017.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Yasameen Hashim Kudhair , Abbas K. Almansorri , Lubna Abdulazeem

This work is licensed under a Creative Commons Attribution 4.0 International License.
Current Clinical and Medical Education













