An Epidemiological Study of TB Infection before and during the COVID-19 Pandemic in Thi-Qar Province
Keywords:
TB Infection, COVID-19, Pandemic, Thi-Qar ProvinceAbstract
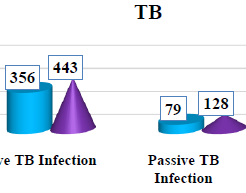
Tuberculosis (TB) is an airborne infectious disease caused by organisms of the Mycobacterium tuberculosis complex. Although primarily a pulmonary pathogen, M. tuberculosis can cause disease in almost any part of the bodyBased on the immunological mechanism involved, a shared dysregulation of immune responses in COVID-19 and TB has been found, suggesting a dual risk posed by co-infection worsening COVID-19 severity and favouring TB disease progression.The results of our study are as follows:Prevalence of TB Infection in Thi-Qar Province during 2022-2023 According to Infection Type .TB infection was active infection during 2022 356 (81.84%)In the same hand, high active TB infection in 2023 was active infection 433 (77.18%).Prevalence of TB Infection in Thi-Qar Province during 2022-2023 According to Months of Infections: was recorded the high TB infection in 2022 was during October.In the other hand, the high TB infection in 2023 was during July while the lowest TB infection was in September in both years.Prevalence of Active TB Infection in Thi-Qar Province during 2022-2023 According to Months of Infections:was recorded the high active TB infection in 2022 was during October, while the lowest active TB infection was in February and September. In the other hand, the high active TB infection in 2023 was during August, while the lowest active TB infection was in October. Prevalence of Active Pulmonary TB Infection According to Months of Infections: was recorded the high pulmonary active TB infection in 2022 was during May, while the lowest active pulmonary TB infection was in September . In the other hand, the high active pulmonary TB infection in 2023 was during August , while the lowest active pulmonary TB infection was in June .Prevalence of Active Out-pulmonary TB Infection:was recorded the high out-pulmonary active TB infection in 2022 was during October, while the lowest active out-pulmonary TB infection was in August. In the other hand, the high active out-pulmonary TB infection in 2023 was during July , while the lowest active out-pulmonary TB infection was in September. Prevalence of Passive TB Infection :was recorded the high passive TB infection in 2022 was during February, while the lowest passive TB infection was in both May and December Iin contrast, the high passive TB infection in 2023 were during both February and Marich,while the lowest passive TB infection was in September in addition, the study not recorded passive infection during April.The current results were noted among 435 recent TB infection during 2022, 10 (2.30%), scored recurrent TB infection, and among 571 TB infection during 2023, 12 (2.10%).
Downloads
References
Algood HM, Lin PL, Yankura D, et al. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J Immunol. 2004; 172: 6846-6857.
Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998; 66: 1277-1281.
Chan J, Xing Y, Magliozzo RS, et al. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates. produced by activated murine macrophages. J Exp Med. 1992; 175: 1111-1122.
Anandaiah A, Dheda K, Keane J, et al. Novel developments in the epidemic of HIV and TB co-infection. Am J Respir Crit Care Med. 2011; 183: 987-997.
Armstrong JA, Hart PD. Phagosome-lysosome interactions in cul- tured macrophages infected with virulent tubercle bacilli: reversal of the usual nonfusion pattern and observations on bacterial sur- vival. J Exp Med. 1975; 142: 1-16.
Austin CM, Ma X, Graviss EA. Common nonsynonymous poly- morphisms in the NOD2 gene are associated with resistance or susceptibility to tuberculosis disease in African Americans. J Infect Dis. 2008; 197: 1713-1716.
Axelrod S, Oschkinat H, Enders J, et al. Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell Microbiol. 2008; 10: 1530-1545.
Bafica A, Scanga CA, Feng CG, et al. TLR9 regulates Thi responses and cooperates with TLR2 in mediating optimal resis- tance to Mycobacterium tuberculosis. J Exp Med. 2005; 202: 1715-1724.
Balasubramanian V, Wiegeshaus EH, Taylor BT, et al. Pathogen- esis of tuberculosis: pathway to apical localization. Tuber Lung Dis. 1994; 75: 168-178.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998; 392: 245-252.
Barry CE III, Lee RE, Mdluli K, et al. Mycolic acids: structure, biosynthesis and physiological functions. Prog Lipid Res. 1998; 37: 143-179.
Abebe F, Holm-Hansen C, Wiker HG, et al. Progress in serodiag- nosis of Mycobacterium tuberculosis infection. Scand J Immunol. 2007; 66: 176-191.
Adams DO. Molecular interactions in macrophage activation. Immunol Today. 1989; 10: 33-35.
Bates JH. Transmission and pathogenesis of tuberculosis. Clin Chest Med. 1980; 1: 167-174.
Chang JC, Miner MD, Pandey AK, et al. igr Genes and Mycobac- terium tuberculosis cholesterol metabolism. J Bacteriol. 2009;191: 5232-5239.
Beatty WL, Rhoades ER, Ullrich HJ, et al. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000; 1: 235-247.
Beckman EM, Porcelli SA, Morita CT, et al. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994; 372: 691-694.
Behar SM, Dascher CC, Grusby MJ, et al. Susceptibility of mice deficient in CDID or TAPI to infection with Mycobacterium tuberculosis. J Exp Med. 1999; 189: 1973-1980.
Behr MA, Wilson MA, Gill WP, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999; 284: 1520-1523.
Alonso S, Pethe K, Russell DG, et al. Lysosomal killing of Myco- bacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci US A. 2007; 104: 6031-6036.
Aly S, Wagner K, Keller C, et al. Oxygen status of lung granulo- mas in Mycobacterium tuberculosis-infected mice. J Pathol. 2006; 210: 298-305.
Benini J, Ehlers EM, Ehlers S. Different types of pulmonary granuloma necrosis in immunocompetent vs. TNFRp55-gene- deficient mice aerogenically infected with highly virulent Mycobacterium avium. J Pathol. 1999; 189: 127-137.
Bermudez LE, Wu M, Petrofsky M, et al. Interleukin-6 antago- nizes tumor necrosis factor-mediated mycobacteriostatic and mycobactericidal activities in macrophages. Infect Immun. 1992; 60: 4245-4252.
Bertagnolli MM, Lin BY, Young D, et al. IL-12 augments antigen-dependent proliferation of activated T lymphocytes. J Immunol. 1992; 149: 3778-3783.
Berthet FX, Lagranderie M, Gounon P, et al. Attenuation of viru- lence by disruption of the Mycobacterium tuberculosis erp gene. Science. 1998; 282: 759-762.
Biedermann T, Mailhammer R, Mai A, et al. Reversal of established delayed type hypersensitivity reactions following therapy with IL-4 or antigen-specific Th2 cells. Eur J Immunol. 2001; 31: 1582-1591.
Brodin P, Rosenkrands I, Andersen P, et al. ESAT-6 proteins: pro- tective antigens and virulence factors? Trends Microbiol. 2004; 12: 500-508.
Eruslanov EB, Lyadova IV, Kondratieva TK, et al. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun. 2005; 73: 1744-1753.
Brosch R, Gordon SV, Marmiesse M, et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci US A. 2002; 99: 3684-3689.
Calmette A, Boquet A, Negre L. Contribution à l'étude du bacille tuberculeux bilié. Ann Inst Pasteur. 1921; 35: 561-570.
Canetti G. Exogenous reinfection and pulmonary tuberculosis a study of the pathology. Tubercle. 1950; 31: 224-233.
Capuano SV III, Croix DA, Pawar S, et al. Experimental Myсо- bacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuber- culosis infection. Infect Immun. 2003; 71: 5831-5844.
Chan J, Fan XD, Hunter SW, et al. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuber- culosis within macrophages. Infect Immun. 1991; 59: 1755-1761.
Bloch H, Segal W. Biochemical differentiation of Mycobacteriumtuberculosis grown in vivo and in vitro. J Bacteriol. 1956; 72: 132-141.
Boshoff HI, Barry CE III. Tuberculosis: metabolism and respira-tion in the absence of growth. Nat Rev Microbiol. 2005; 3: 70-80. 27. Bowdish DM, Sakamoto K, Kim MJ, et al. MARCO, TLR2, andCD14 are required for macrophage cytokine responses to myco- bacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009; 5: e1000474.
Derrick SC, Morris SL. The ESAT6 protein of Mycobacterium tuberculosis induces apoptosis of macrophages by activating cas-pase expression. Cell Microbiol. 2007; 9: 1547-1555.
Diamond JM. Guns, Germs, and Steel: The Fates of Human Soci- eties. New York: W. W. Norton; 1997.
Chegou NN, Black GF, Kidd M, et al. Host markers in Quanti- FERON supernatants differentiate active TB from latent TB infection: preliminary report. BMC Pulm Med. 2009; 9: 21.
Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through Toll- like receptors. Science. 1999; 285: 732-736.
Brindle RJ, Nunn PP, Githui W, et al. Quantitative bacillary response to treatment in HIV-associated pulmonary tuberculosis. Am Rev Respir Dis. 1993; 147: 958-961.
Chen CY, Huang D, Wang RC, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009; 5: e1000392.
Clemens DL, Horwitz MA The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exo- genously administered transferrin. J Exp Med. 1996; 184: 1349-1355.
Cooper AM, Segal BH, Frank AA, et al. Transient loss of resis- tance to pulmonary tuberculosis in p47(phox-/-) mice. Infect Immun. 2000; 68: 1231-1234.
D'Souza S, Denis O, Scorza T, et al. CD4+ T cells contain Myco- bacterium tuberculosis infection in the absence of CD8+ T cells in mice vaccinated with DNA encoding Ag85A. Eur J Immunol. 2000; 30: 2455-2459.
Daffe M, Draper P. The envelope layers of mycobacteria with refer- ence to their pathogenicity. Adv Microb Physiol. 1998; 39: 131-203.
Dannenberg AM. Pathophysiology: basic aspects. In: Schlossberg D, ed. Tuberculosis and Nontuberculous Mycobacterial Infec- tions. 4th ed. Philadelphia, PA: WB Saunders; 1999: 17-47.
Filipe-Santos O, Bustamante J. Haverkamp MH, et al. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J Exp Med. 2006; 203: 1745-1759.
De Backer Al, Mortele KJ, De Keulenaer BL., et al. Tuberculosis: epidemiology, manifestations, and the value of medical imaging in diagnosis. JBR-BTR. 2006; 89: 243-250.
de Chastellier C, Forquet F, Gordon A, et al. Mycobacterium requires an all-around closely apposing phagosome membrane to maintain the maturation block and this apposition is re-established when it rescues itself from phagolysosomes. Cell Microbiol. 2009; 11: 1190-1207.
de Valliere S, Abate G, Blazevic A, et al. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun. 2005; 73: 6711-6720.
Delgado MA, Elmaoued RA, Davis AS, et al. Toll-like receptors control autophagy, EMBO J. 2008; 27: 1110-1121.
Fantuzzi G. Dinarello CA. The inflammatory response in interleukin-1 beta-deficient mice: comparison with other cytokine-related knock-out mice. J Leukoc Biol. 1996; 59: 489-493.
Fenhalls G, Wong A, Bezuidenhout J, et al. In situ production of gamma interferon, interleukin-4, and tumor necrosis factor alpha mRNA in human lung tuberculous granulomas. Infect Immun. 2000; 68: 2827-2836.
Denis M. Human neutrophils, activated with cytokines or not, do not kill virulent Mycobacterium tuberculosis. J Infect Dis. 1991; 163:919-920.
Denis M, Gregg EO. Recombinant interleukin-6 increases the intracellular and extracellular growth of Mycobacterium avium. Can J Microbiol. 1991; 37: 479-483.
Fischer K, Scotet E, Niemeyer M, et al. Mycobacterial phospha- tidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci US A. 2004; 101: 10685-10690.
Dong C. Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol Rev. 2008; 226: 80-86.
Dorman SE, Holland SM. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000; 11: 321-333.
Dussurget O, Stewart G, Neyrolles O, et al. Role of Mycobacter- ium tuberculosis copper-zinc superoxide dismutase. Infect Immun. 2001; 69: 529-533.
Eum SY, Kong JH, Hong MS, et al. Neutrophils are the predomi- nant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010; 137: 122-128.
Ferrari G, Langen H, Naito M, et al. A coat protein on phago- somes involved in the intracellular survival of mycobacteria. Cell. 2019 97: 435-447.
Artman M, Bekierkunst A, Goldenberg I. Tissue metabolism in infection: biochemical changes in mice treated with cord factor. Arch Biochem Biophys. 2020 105: 80-85.
Fine PE, Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 2021; 346: 1339-1345.
Divangahi M, Mostowy S, Coulombe F, et al. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol. 2022; 181: 7157-7165.
Diveu C, McGeachy MJ, Cua DJ. Cytokines that regulate autoim- munity. Curr Opin Immunol. 2020; 20: 663-668.
Comstock GW. Epidemiology of tuberculosis. Am Rev Respir Dis, 2021; 125: 8-15.
Cooper AM, Roberts AD, Rhoades ER, et al. The role of interleukin-12 in acquired immunity to Mycobacterium tubercu- losis infection. Immunology. 2019; 84: 423-432.
Mohammad Hadi, Ahmed Methab Athab, and Rokan M. Fadhil. "Evaluation of Auto-antibody Profiles in Patients with Tuberculosis." Diyala Journal of Medicine 15.1 (2018): 50-60.
Sultan, Hassan I., and Mohemid M. Al-Jebouri. "Pulmonary tuberculosis in Alzab district." Tikrit Medical Journal 16.1 (2010): 37-41.
Sultan, H. I., & Al-Jebouri, M. M. (2010). Pulmonary tuberculosis in Alzab district. Tikrit Medical Journal, 16(1), 37-41.
Faris, M. H., Athab, A. M., & Fadhil, R. M. (2018). Evaluation of Auto-antibody Profiles in Patients with Tuberculosis. Diyala Journal of Medicine, 15(1), 50-60.
Mohammad Hadi, Ahmed Methab Athab, and Rokan M. Fadhil. "Evaluation of Auto-antibody Profiles in Patients with Tuberculosis." Diyala Journal of Medicine 15, no. 1 (2018): 50-60.
Harvard Faris, M.H., Athab, A.M. and Fadhil, R.M., 2018.
Evaluation of Auto-antibody Profiles in Patients with Tuberculosis. Diyala Journal of Medicine, 15(1), pp.50- 60.
Faris MH, Athab AM, Fadhil RM. Evaluation of Auto- antibody Profiles in Patients with Tuberculosis. Diyala Journal of Medicine. 2018 Oct 8;15(1):50-60.
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Current Clinical and Medical Education













